PharmNXT Biotech’s Negative Pressure Rigid Wall Isolators system “NXT Rigid-N” is a high-quality containment isolator system, specially designed for creating controlled negative pressure inside the isolator. It also can effectively control & monitor operating parameters and safeguard the operator & the surroundings from the highly potent products.
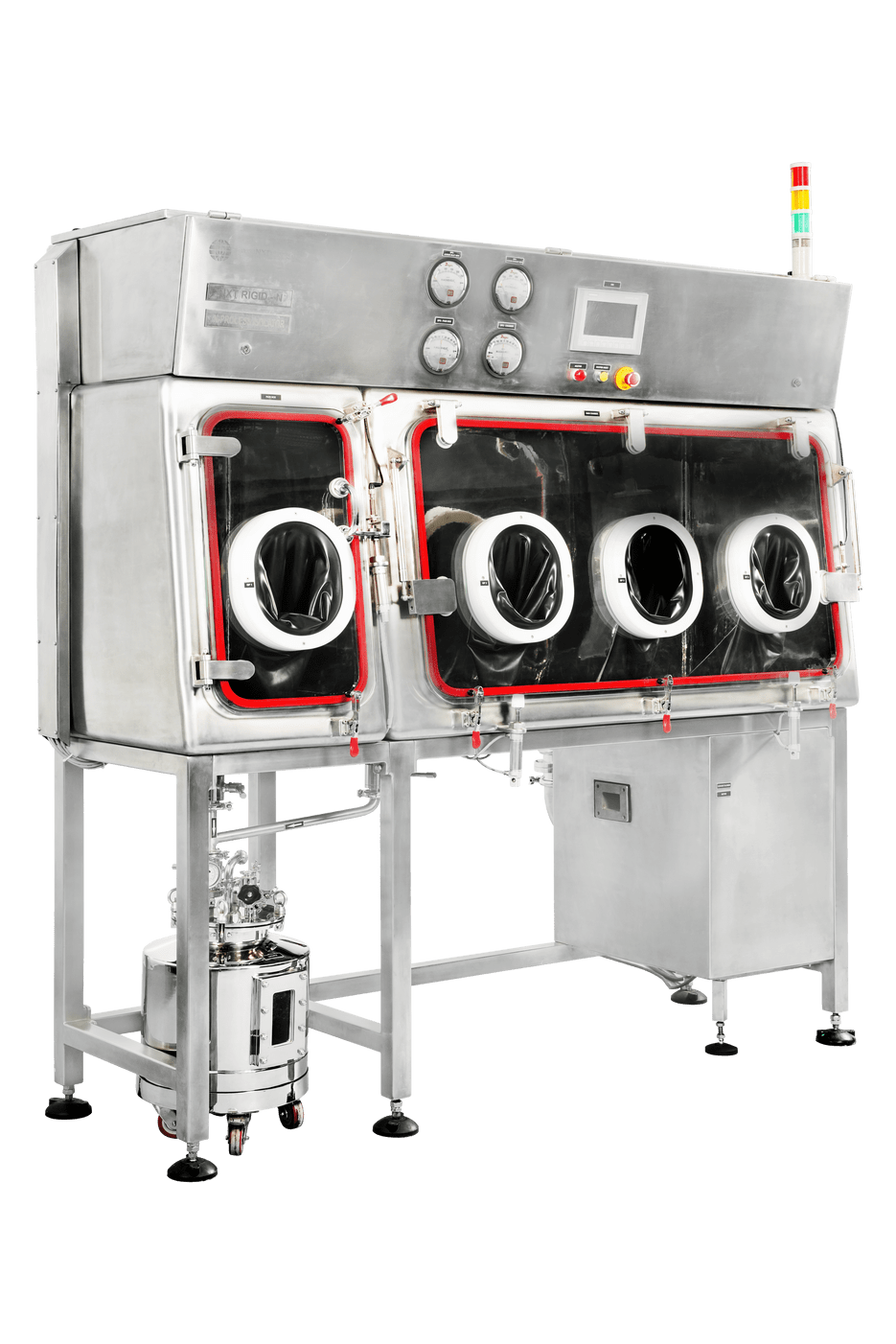
NXT Rigid-N systems are designed to meet the challenges and market requirements for low OEL containment systems in the Pharmaceutical, Biotechnology, and formulation industries. The concept of isolator technology, which is well-known in the pharmaceutical industry, protects the process from the operator and or the operator.
Although the development and improvement of the isolator technology are continuous, it should be noted that the most significant changes have been in the form of its increased acceptance, backed by guidelines and standards produced by regulatory bodies.
Modular Concept
The platform design allows easy customization of the process chromatography system. One or more systems that meet your precise process requirements can be produced by choosing from the required options. You can choose from a variety of sensors and specify the number of valves and ports you need.
Options to choose from:
-
- Additional inlet and outlet valves
- Inline filter housing (1 or 2)
- Pressure sensor (before filter or after column)
- Air sensor (after bubble trap, before pump, or both)
- Additional conductivity and temperature sensors before column)
- Additional pH sensor (before column)
- Sampling valve (after column)
- Position detectors for valves
- Steam-in-place (SIP)
- Clean-in-place (CIP)
- Gradient function (controlled either by flow rate only or by flow rate and conductivity)
Each option includes the required combination of hardware and software modules. All standard features and options have been validated on the platform.
User-Friendly Software:
The control unit uses a simple, user-friendly interface via the touchscreen for data input and programming commands. The system is shielded from unauthorized access with password protection (four levels), and all events and actions (alarms, process steps, manual commands, etc.) are recorded in accordance with cGMP guidelines. The program allows you to operate the system manually or automatically.
The automatic mode has:
-
- Multiple operations
- Configurable fluid paths
- Control of inlets/outlets and column valve
- Multiple end conditions
- Pause and hold alarms
- Interactive pausing steps
- Full trend review, trend analysis, and printing from the system are all standard. Data export and configurable interfacing to external software is also included.
Hygienic Design:
All process piping and valves are made from Stainless Steel 316L (ASTM 1.4404) to conform to ASME BPE requirements. Only membrane valves have been used for the process flow path. Block body valves, compact instruments, and other devices have been used to minimize the holdup volumes. Cables and pneumatic lines are run through stainless steel conduits protecting them from damage or contamination.
The NXTchrom system can be cleaned in place with NaOH. The option for automated cleaning in place is available on request which includes a specialized program with additional valves. A Steam in Place system option is also available upon request.
MORE SOLUTIONS

NXTtff
The NXT TFF system by PharmNXT Biotech is a system that’s perfect for running fully automated UF/DF processes at high capacity. It can handle any volume between 20L and 500L (200 g to 2.5 kg), and the compact system, has a low hold-up volume and the capacity to accommodate cassettes with surface area ranging from 0.5 to 2.5 m2

Chromatography Resins (Purolite)
The purification of commercially-available monoclonal antibodies (mAbs) on the market today is typically performed in three chromatography steps. Purolite offer industry-leading solutions for each step.
Protein A Affinity Chromatography
A Protein A affinity resin is utilized as an initial antibody capture step. Praesto Protein A resins deliver exceptionally high purity (>99%) and yield in a single, efficient step.
Cation Exchange Chromatography
A Sulphopropyl- (SP) functionalized cation exchange resin in ‘bind and elute’ mode is used to remove aggregates and HCP (Host Cell Proteins).
Anion Exchange Chromatography
A Quarternary Amine (Q) functionalized anion exchange resin is used in ‘flow-through’ mode to remove trace contaminates and ensure sufficient viral clearance.

NXTmfilt
Single-use technology continues to be adopted for many areas of bioprocessing. The use of automation provides additional benefits in manufacturing such as consistency in product quality, reduced labor costs, and reduction of operator errors.


