


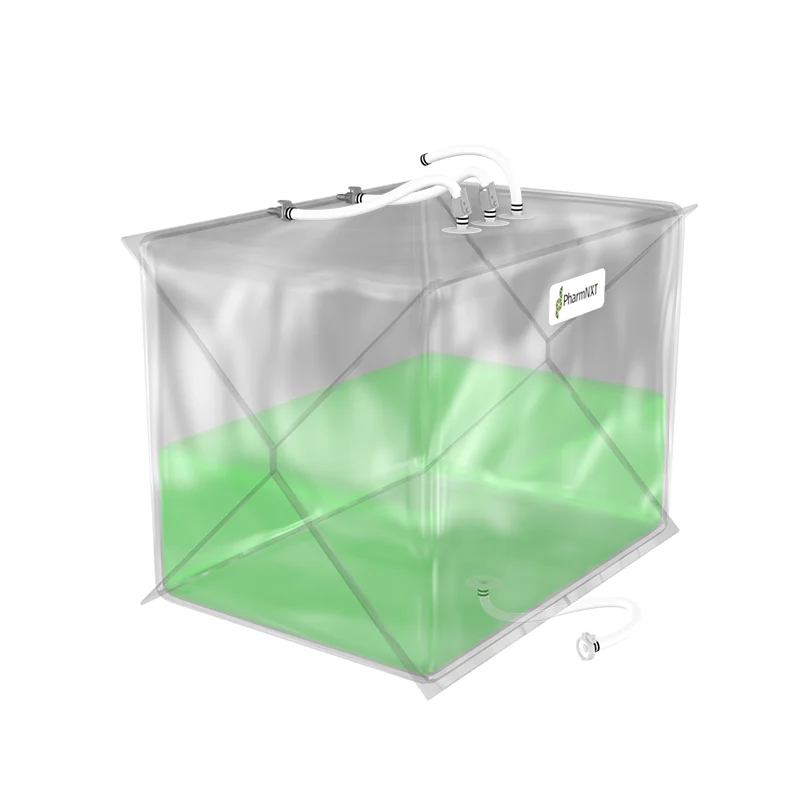




The XELTA 3D bags are designed for reliable, flexible, and easy-to-install large-scale applications in biopharmaceutical and pharmaceutical manufacturing processes. They offer state-of-the-art design features that improve single-use system robustness and ease of use. With various types and specifications available, these bags are suitable for storage, transport, filtering, and mixing of biopharmaceutical liquids. They comply with pharmacopeia standards and undergo extensive testing to ensure quality and biocompatibility. The specialized film used in the bags provides good resistance to chemicals and solvents and has excellent clarity, tensile strength, and permeability properties. Gamma irradiation is recommended for sterilization.
XELTA 3D bags have been designed for safe processing and storage of a wide range of biopharmaceutical liquids in a variety of applications such as:
The following tests are conducted for Pharmacopoeia compliance and Bio-compatibility.
USP <85> Bacterial Endotoxins – LAL test
USP <87> Biological Reactivity Test in-vitro
USP <88> Biological reactivity testing, in-vivo, Class VI European Pharmacopoeia tests 3.1.5
USP <661.1> Polyethylene Physiochemical Tests, Extractable Metals, Plastic Additives
The film used in XELTA 3D bags is specialized for biopharmaceutical and pharmaceutical processes.
The thickness of the film is 0.325mm
The recommended sterilization method is Gamma Irradiation. The dose for gamma sterilization is 25-50 KGy
Good resistance to many chemicals
Good resistance to polar solvents (alcohols, esters, etc)
Water pH compatibility – 2.5 to 11
Clarity – ASTM D-1003
Tensile Strength at break – ASTM D-882
O2 permeability – ASTM D-3985
CO2 permeability – ASTM F-2476



