




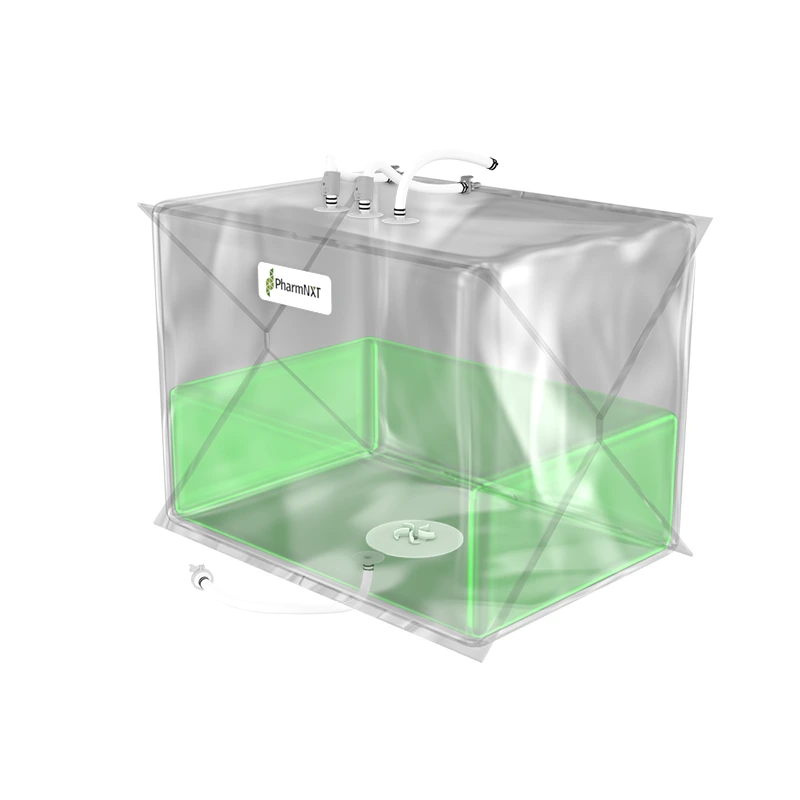
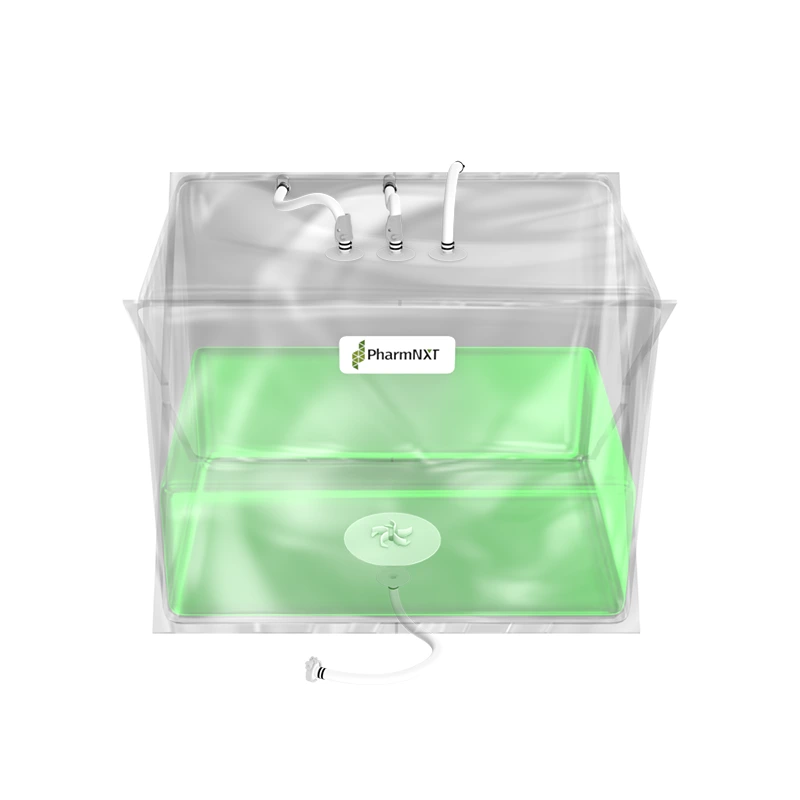
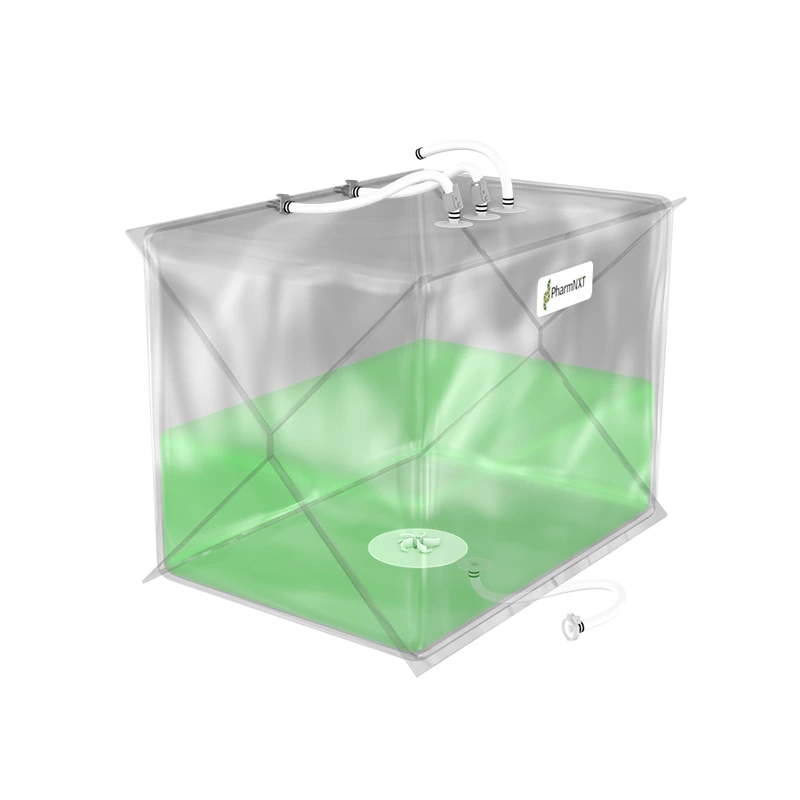










The XELTA Mix bags are designed for reliable and flexible large-scale applications in biopharmaceutical and pharmaceutical manufacturing. Made from premium film, these bags offer ease of use and efficient product recovery for handling large fluid volumes. They are available in various sizes and can be used for buffer, media, intermediate, or drug substance mixing. Customizable configurations are available to meet specific process requirements. Manufactured under ISO 9001:2015 certified quality management systems and cGMP conditions, the bags undergo extensive testing to ensure compliance with pharmacopoeia standards and bio-compatibility. The specialized multilayer film used in XELTA Mix bags provides excellent chemical resistance, including polar solvents, and has favorable properties such as clarity, tensile strength, and gas permeability. Gamma irradiation is the recommended sterilization method for these bags.
The XELTA Mix bags allow users to order a wide range of configurations with a choice of connectors, tubing, clamps, etc., from any supplier to configuring the bags as per process requirements.
The XELTA Mix bags are designed, developed and manufactured in accordance with an ISO 9001:2015 certified Quality Management System. They undergo extensive testing before shipping. We make sure our products are manufactured according to cGMP under ISO7 classroom conditions.
Various tests are conducted for Pharmacopoeia compliance and Bio-compatibility.
USP <85> Bacterial Endotoxins – LAL test
USP <87> Biological Reactivity Test in-vitro
USP <88> Biological reactivity testing, in-vivo, Class VI European Pharmacopoeia tests 3.1.5
USP <661.1> Polyethylene Physicochemical Tests, Extractable Metals, Plastic Additives
The film used in XELTA Mix bags is specialized for biopharmaceutical and pharmaceutical processes.
The film chosen is a multilayer film with ULDPE as a product contact layer and PE outer layer with gas barriers in between.
The thickness of the film is 0.325mm
The recommended sterilization method is Gamma Irradiation. The dose for gamma sterilization is 25-50 KGy
Good resistance to a wide range of pharma and biopharma-grade chemicals
Good resistance to polar solvents (alcohols, esters, etc)
Water pH compatibility – 2.5 to 11.
Clarity – ASTM D-1003
Tensile Strength at break – ASTM D-882
O2 permeability – ASTM D-3985
CO2 permeability – ASTM F-2476



